Compliance Program: Difference between revisions
No edit summary |
mNo edit summary |
||
| Line 17: | Line 17: | ||
</table> | </table> | ||
<br /> | <br /> | ||
[[Compliance Program]] | [[Compliance Hotline]] | [[Investigations by Third Parties]] | [[Research Integrity]] | [[Export Control]] | [[Code of Conduct]] | [[Use of Human Anatomical Material]] | [[Clinical Trial Professional and Technical Fee Billing]] | [[Contracts]] | [[Conflict of Interest]] | [[Red Flag Identity Theft Prevention Program]] | [[Principles of Financial Stewardship]] | [[Human Tissue Use and Transfer]] | [[International Research Policy]] | [[Health Care Vendor Interactions | [[Compliance Program]] | [[Compliance Hotline]] | [[Investigations by Third Parties]] | [[Research Integrity]] | [[Export Control]] | [[Code of Conduct]] | [[Use of Human Anatomical Material]] | [[Clinical Trial Professional and Technical Fee Billing]] | [[Contracts]] | [[Conflict of Interest]] | [[Red Flag Identity Theft Prevention Program]] | [[Principles of Financial Stewardship]] | [[Human Tissue Use and Transfer]] | [[International Research Policy]] | [[Health Care Vendor Interactions]] | [[Credit Hour Definition]] | ||
<br /><br /> | <br /><br /> | ||
Policy No.: '''8000'''<br /> | Policy No.: '''8000'''<br /> | ||
Revision as of 10:14, December 1, 2017
| Human Resources | Safety/Security | Research Compliance | Compliance | Privacy/Information Security | Business Operations | Intellectual Property |
Compliance Program | Compliance Hotline | Investigations by Third Parties | Research Integrity | Export Control | Code of Conduct | Use of Human Anatomical Material | Clinical Trial Professional and Technical Fee Billing | Contracts | Conflict of Interest | Red Flag Identity Theft Prevention Program | Principles of Financial Stewardship | Human Tissue Use and Transfer | International Research Policy | Health Care Vendor Interactions | Credit Hour Definition
Policy No.: 8000
Effective Date: 11/01/06
Revised Date: 03/31/17
Reviewed Date: 03/31/17
Compliance Program Policy
Basis for Policy
The purpose of the Compliance Program and Committee is to identify UNMC’s compliance risks, as well as make recommendations and implement solutions, policies, procedures and standards of conduct to ensure that UNMC complies with all applicable federal, state and local laws and regulations. In support of this purpose, UNMC’s Compliance Program will designate a compliance officer and compliance committee whose responsibilities will include the following: to conduct effective training and education, develop effective lines of communication, and conduct internal monitoring and auditing for adherence to policies and procedures. The University of Nebraska Medical Center (UNMC) Compliance Program consistent with UNMC Policy No. 8006, Code of Conduct, mandates that all UNMC faculty, staff, and students comply with: all applicable federal, state and local laws; all relevant regulations; and University of Nebraska and UNMC Policies and Procedures.
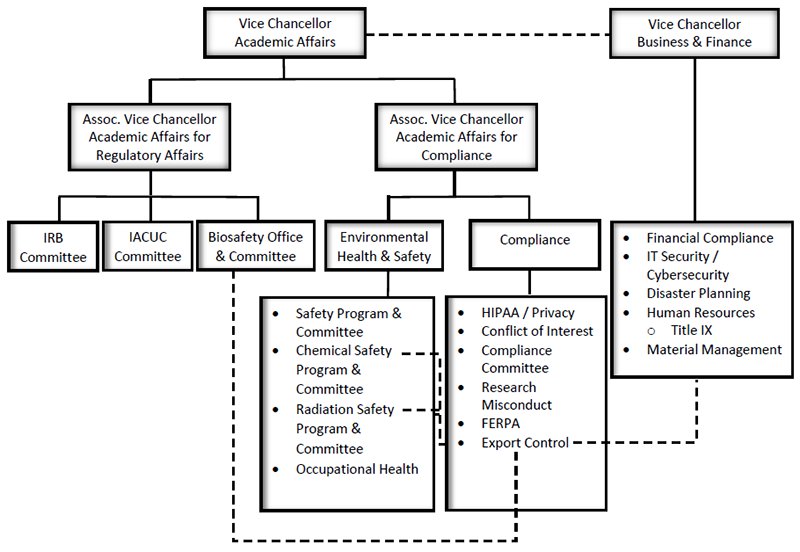
Compliance Program Structure
Associate Vice Chancellor for Compliance
The Associate Vice Chancellor for Compliance reports directly to the Vice Chancellor for Academic Affairs and is responsible for overseeing the development and implementation of policies, procedures and practices necessary to effect compliance with federal, state, and local laws and regulations.
Compliance Officer
The Compliance Officer shall report to the Associate Vice Chancellor of Academic Affairs for Compliance, but has the authority to communicate directly with the Chancellor or Vice Chancellors for Academic Affairs, Business and Finance, Research, Deans or others on compliance matters which include, but are not limited to:
- coordinate with subject matter experts to analyze laws and regulations applicable to UNMC to ensure compliance;
- monitor day-to-day compliance activities;
- develop, initiate, maintain and revise policies and procedures for compliance with laws and regulations applicable to UNMC;
- oversee visits by regulatory agencies and responses to inquiries and investigations;
- report compliance matters directly to the UNMC Chancellor’s Council;
- coordinate with the Associate General Counsel for Health Care on legal issues;
- coordinate with the Director of Internal Audit;
- coordinate with the Information Security Officer;
- respond to compliance hotline calls;
- disseminate information to support compliance and training on the Compliance Program;
- chair the compliance committee.
Compliance Committee
The Compliance Committee assists the Compliance Officer with the above duties and helps manage the Compliance Program. The Compliance Committee shall seek to leverage the compliance efforts of each area and avoid duplication of effort. The Compliance Committee shall analyze UNMC’s key risk areas, and monitor and promote compliance. The Compliance Committee shall oversee and monitor department response to identified risks as identified by the Internal Audit Director’s annual audit plan as well as data generated through monitoring and individual reporting.
The Compliance Committee shall meet no less than quarterly. The Compliance Committee shall be composed of at least the following representatives who shall be responsible for the overseeing compliance and assist the Compliance Officer with managing the Compliance Program:
- Compliance Officer
- Associate General Counsel for Health Care
- Export Control Compliance Officer
- Human Resources Director
- Information Security Officer
- Chair of the Institutional Review Board
- Executive Director, Environmental Health & Safety
- Intellectual Property Director
- Internal Audit Director
- Nebraska Medicine Compliance Officer or Compliance Representative for Nebraska Medicine
- Privacy Officer
- Controller
- Sponsored Programs Administration
- Sponsored Programs Accounting
- Director of Procurement
- Chief Student Affairs Officer
The Compliance Committee or Compliance Officer may request reports annually from certain representatives and others to address compliance issues as necessary. The purpose of the reports will be to:
- develop policies related to compliance;
- establish compliance training programs;
- assist with identifying areas of potential compliance vulnerabilities;
- assist with understanding the compliance risks and related regulations and laws;
- assist with response to alleged violation of rules, regulations, policies, procedures and other functions as needed to enforce this policy.
Representatives would be from the following areas:
- NU Director of Internal Audit
- Biosafety
- College of Dentistry
- College of Medicine
- College of Nursing
- College of Pharmacy
- College of Public Health
- College of Allied Health
- UNMC Physicians
- Comparative Medicine/Institutional Animal Care and Use Committee
- Eppley Institute/Fred & Pamela Buffett Cancer Center
- Facilities Management
- Munroe-Meyer Institute
- Environmental Health & Safety
- Student Services
- Others as necessary
Compliance Responsibilities
Compliance Areas
Compliance responsibilities shall be established per UNMC and/or Board of Regents policies and procedures linked and referenced below:
| Research Compliance | |
| Code of Conduct | UNMC Policy No. 8006, Code of Conduct |
| IRB | Institutional Review Board |
| IACUC | Institutional Animal Care and Use Committee, BOR Policy 3.2.8 |
| Conflict of Interest | UNMC Policy No. 8010, Conflict of Interest |
| Clinical Trial Billing | UNMC Policy No. 8008, Clinical Trial Professional and Technical Fee Billing |
| Research Integrity | UNMC Policy No. 8003, Research Integrity |
| Sponsored Programs Administration and Sponsored Programs Accounting | UNMC Policy No. 3001, Sponsored Programs, UNMC Policy 6100, Direct Cost, UNMC Policy 6101, Facilities and Administrative, F&A) Cost, UNMC Policy 6102, Institutional Base Salary, UNMC Policy 6103, Unallowable Cost, UNMC Policy 6104, Sponsored Project Cost Share, UNMC Policy 6105, Effort Certification, UNMC Policy 6106, Cost Transfer, UNMC Policy 6107, Service Center, UNMC Policy 6108, Subrecipient |
| Environmental and Safety Compliance | |
| Safety | UNMC Environmental Health & Safety Department, UNMC Policy No. 2000, Safety |
| Radiation Safety | UNMC Environmental Health & Safety Department |
| Bloodborne Pathogens | Infection Control, UNMC Policy No. 2004, Bloodborne Pathogens Exposure |
| Biosafety | Institutional Biosafety Committee, UNMC Policy No. 2005, Waste Handling, Biohazardous Waste Handling |
| Chemical Safety | UNMC Environmental Health & Safety Department, UNMC Policy No. 2002, Shipment of Hazardous Materials or Dangerous Goods |
| Security | UNMC Campus Security |
| Intellectual Property | |
| Copyright | UNMC Policy No. 6036, Reproducing Copyrighted Materials |
| Privacy and Information Security | |
| Privacy, Confidentiality and Information Security | UNMC Policy No. 6045, Privacy, Confidentiality and Information Security |
| Use and Disclosure of Protected Health Information | UNMC Policy No. 6054, Use and Disclosure of Protected Health Information |
| Retention/Destruction/Disposal of Private/Confidential Information | UNMC Policy No. 6056, Retention and Destruction/Disposal of Private and Confidential Information |
| Other | |
| Computer Use and Information Security | UNMC Policy No. 6051, Computer Use and Electronic Information Security |
| Export Control | UNMC Policy No. 8005, Export Control |
| Human Resources | Human Resources |
| Internal Audit | UNMC Policy No. 8016, Internal Audit |
| Student Services | Student Services, Student Handbook Compliance Matrix, FERPA Information |
| Title IX | UNMC Policy No. 1099, Non-Discrimination and Harassment, UNMC Policy No. 1107, Sexual Misconduct |
| Family Educational Rights and Privacy Act (FERPA) | Student Services, FERPA Information |
| False Claims Act | Contact the Chief Compliance Officer (see also the UNMC Compliance Hotline 1-866-568-5430), False Claims Act 31 U.S.C. §§ 3729-3733 |
| Foreign Corrupt Practices Act | Contact the Chief Compliance Officer (see also the UNMC Compliance Hotline 1-866-568-5430), Foreign Corrupt Practices Act (FCPA) 15 U.S.C. §§ 78dd-1, et seq. |
Compliance Committee Meetings
The purpose of Compliance Committee meetings shall be to analyze, assess, manage, coordinate and develop corrective action(s) related to compliance risks identified for the purpose of compliance with UNMC’s Code of Conduct, University of Nebraska and UNMC Policies and Procedures.
The Compliance Office shall complete meeting minutes and maintain them on file for no less than seven years. The draft meeting minutes shall be distributed to committee members, vice chancellors, college deans and executive directors to inform UNMC leadership about compliance activities.
Compliance Hotline
A UNMC compliance hotline has been established to provide individuals with an additional communication channel to report compliance concerns. The Compliance Officer shall investigate concerns and take corrective action in response to identified issues. For additional information, see UNMC Policy No. 8001, Compliance Hotline.
Compliance Training
All University of Nebraska Medical Center employees, students (including visiting students), and faculty (including but not limited to courtesy, adjunct, volunteers) must be knowledgeable of and comply with laws and regulations related to their duties or field of study as determined by the Compliance Officer and Compliance Committee.
Mandatory compliance training is required of UNMC staff and students. It is the individual's responsibility to complete the compliance training requirements at http://www.unmc.edu/academicaffairs/compliance/training-requirements/index.html. Failure to complete the training requirements will be grounds for corrective action up to and including dismissal or termination of employment. Delinquency notices will be sent according to the guidelines below:
| Days Overdue (days past deadline date) | Employees | Students |
|---|---|---|
| 30 Days | E-mail/verbal notice from unit management | E-mail/verbal notice from Dean’s office |
| 60 Days | Communication from Human Resources | Letter from Vice Chancellor for Academic Affairs; records placed on hold |
| 90 Days | Notification to Dean, access removed from all UNMC resources except Blackboard until training has been completed |
Statement of Understanding
All employees and students shall sign a Statement of Understanding at the beginning of employment/start of school and annually thereafter, documenting that they have read, understand and agree to adhere to policies on: code of conduct, non-discrimination, sexual harassment; privacy and information security; and drug-free workplace.
Additional Information
- Contact the Chief Compliance Officer
- For a complete listing of Contacts and Compliance Topics see the Compliance Matrix for details.
- UNMC Policy No. 1003, Drug Free Campus
- UNMC Policy No. 1099, Non-Discrimination and Harassment
- UNMC Policy No. 2002, Shipment of Hazardous Materials or Dangerous Goods
- UNMC Policy No. 2004, Bloodborne Pathogens Exposure
- UNMC Policy No. 6036, Reproducing Copyrighted Materials
- UNMC Policy No. 6045, Privacy, Confidentiality and Information Security
- UNMC Policy No. 6051, Computer Use and Electronic Information Security
- UNMC Policy No. 8001, Compliance Hotline
- UNMC Policy No. 8005, Export Control
- UNMC Policy No. 8006, Code of Conduct
- UNMC Policy No. 8010, Conflict of Interest
This page maintained by dkp.